I am currently working on understanding the various aspects of hydrogen incorporation in garnet.
As garnet is a nominally anhydrous mineral (no –OH neither H2O groups takes place in the structural formula), if hydrogen sits in the crystal structure, it has to substitute to other cations.
- Where does sit hydrogen in the garnet crystal lattice? Is there several sites that can accommodate hydrogen in the structure?
- How stable is hydrogen incorporated in garnet over the long metamorphic history of garnet bearing rocks?
- How much hydrogen is stored in garnet from deeply subducted crust?
To answer these questions I am carrying a multi-step/multi-method study.
- Hydrogen diffusion experiments and hydrous garnet endmember synthesis.
Diffusion experiments (Status: done – published). I carried H diffusion experiments on natural gem-quality garnets, Grs90And9Alm1 and nearly pure Spessartine. Experiments were conducted on a range of temperature from 750 °C to 1050 °C in air (adapted duration from 10d to 2h30), and at 850 °C for 3 days for lower oxygen fugacities, from ΔQFM +8 to ΔQFM-3. Results shows a different behaviour of the OH stretching infrared peaks regarding H extraction suggesting that different point defects occurs in the garnet structure. In addition, hydrogen appears to diffuse very fast in air but slow down with lower oxygen fugacity highlighting an oxidation related mechanism where one 2+ cation (Fe2+ or Mn2+) gets oxidised and extra charge in compensated by one H+ going out of the crystal structure.
Fe2+ + H+ + ¼O2 = Fe3+ + ½ H2O
For more details, you can download my article here.
Fig 1: (a) Optical map and (b) high resolution Fourier transform infrared spectroscopy map of a grossular garnet slice after diffusion experiment at 850 °C in air for 5 days. The 300 µm thick slice was taken in the centre of a 1.5mm side cube which underwent the experiment. The change of colour at the rim on the optical image (a) is due to iron oxidation.
Hydrous garnet endmember synthesis (Status: Ongoing – first experiments in nov. 2019). I am conducting synthesis experiments using hydrothermal cold-seal type vessels. Oxide-hydroxide powders are mixed in stoichiometric proportions for grossular or spessartine compositions, then introduced in gold capsules and run at 700 °C and 2kBar for a duration of 2 weeks. The aim is to synthetise pure endmembers with hydrous point defects and observe the peak OH stretching infrared peak positions, and, in a second step, dop these garnets with various elements and see the influence on the peak positions and the possible occurrence of new peaks.
- Natural sample analysis: determination of hydrogen content in garnets from various environments.
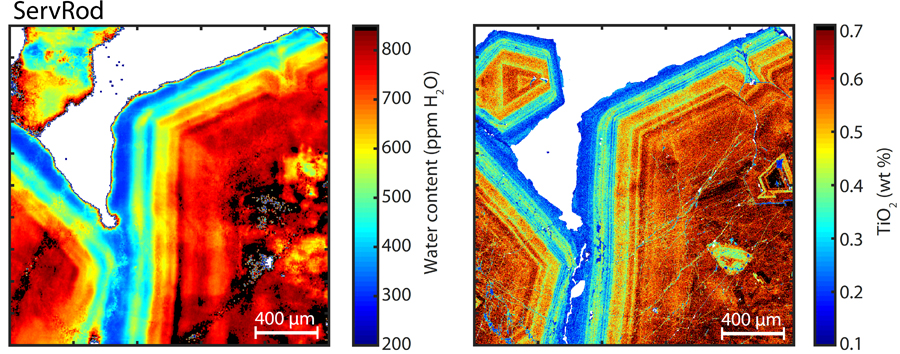
– HP subducted crust, the case of Zermatt area (Status: article in preparation). I have been sampling all different garnet bearing rocks present in Zermatt area, South Switzerland. These rocks are interpreted as fragments of a former oceanic crust and its sedimentary cover. OH content has been investigated for all sample as an attempt to understand the relationships between hydrogen incorporation in garnet and garnet compositions, pressure and temperature conditions. The study enables to compute a hydrogen budget in garnets of deeply subducted crust.
– HT metamorphism on oceanic crust, the case of Valmalenco (Status: analysis ongoing). I sampled during my PhD various sample of garnet bearing rocks in the Valmalenco valley. The garnet bearing rocks present in Valmalenco consists mainly of rodingites and meta-sedimentary rocks cooked by late intrusion of tonalite during the later stage of alpine orogeny.
– “Monster” project: various samples of garnet, from hydrothermal to high pressure environments.

Fig. 4: From left to right, Spessartine on quartz, Yunxiao county, Zhangzhou, China; Grossular, Asbestos mine, Quebec, Canada; Grossular, Xalostue, Mexico

Fig. 5: Double polished epoxy mounts of various garnets used EPMA and FTIR analysis. Samples were bought at a gem shop or sampled from the collection of the museum of natural history in Bern.
I focus especially on very “special” gem quality garnets: nearly pure endmembers & unusual compositions to investigate hydrogen incorporation as a function of chemistry, and OH stretching region peak positions in mid-infrared spectra.
In complement of Fourier transform infrared spectroscopy I performed ionic microprobe analysis on SIMS and SHRIMP to cross calibrate these methods and investigate potential matrix effects between different endmembers.
– Development of computer programs to extract the best data from FTIR analysis: automatic spectral deconvolution (identifying contribution of different peaks to an observed complex FTIR spectra), and image processing for FTIR high resolution spectroscopy (FPAMaps, StackMaps) (article ready to submit).
- 2D diffusion modelling: a way to construct relationships between experimental results and natural samples characterisation.
I realised some diffusion modelling in 2D to bring face to face diffusion coefficients determined from experiments with OH zoning observed in HP metamorphic garnets. The results highlight a paradox between these two sets of data: hydrogen diffusion appears to be too fast to explain retention in HP metamorphic garnets. Explanation may come from coupled substitution that would stabilise hydrogen in the garnet structure. (article ready to submit).
Example: with a grossular garnet from HP rodingite (same as fig.6). If we interpolate the diffusion coefficient at metamorphic peak temperature for the fast oxidation related mecanism. we obtain a log D=16.6 and the garnet would loose all hydrogen in less than 500 years. Even with interpolated coefficient for the low oxygen fugacity mechanism, we cannot explain the preservation of water zoning through metamorphic history. The diffusion coefficient has to be as low as logD=-24.1 to explain the preservation of the water zoning over 4M years (max duration around peak conditions). See videos: